- 9 months ago
- 21Minutes
- 4612Words
- 414Views
You may have heard the term ‘leaky gut’ talked about in recent times. It has become more of a common occurrence and is now widely accepted as a cause of both inflammation and autoimmune disturbances.
(1) Leaky Gut As a Danger Signal for Autoimmune Diseases. Pubmed https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5440529/
(2) Immunoglobulin A (IgA). Britich Society for Immunology. https://www.immunology.org/public-information/bitesized-immunology/receptors-and-molecules/immunoglobulin-iga
(3) Alterations in Intestinal Permeability: The Role of the “Leaky Gut” in Health and Disease. PUBMED https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6467570/
(4) Intestinal Barrier Function: Molecular Regulation and Disease Pathogenesis. PUBMED https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4266989/
(5) Gastrointestinal Candida colonisation promotes sensitisation against food antigens by affecting the mucosal barrier in mice. PUBMED https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1856330/
(6) Alterations in intestinal permeability. PUBMED https://www.ncbi.nlm.nih.gov/pmc/articles/PMC1856434/
(7) Intestinal permeability – a new target for disease prevention and therapy. PUBMED https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4253991/
(8) Dietary fructose and intestinal barrier: potential risk factor in the pathogenesis of nonalcoholic fatty liver disease. PUBMED https://www.ncbi.nlm.nih.gov/pubmed/19679262/
(9) Comparison with ancestral diets suggests dense acellular carbohydrates promote an inflammatory microbiota, and may be the primary dietary cause of leptin resistance and obesity. PUBMED https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3402009/
(10) The efficacy of probiotics in the treatment of irritable bowel syndrome: a systematic review. https://gut.bmj.com/content/59/3/325.short
(11) A systematic review and meta-analysis: probiotics in the treatment of irritable bowel syndrome. PUBMED https://www.ncbi.nlm.nih.gov/pubmed/19220890
(12) Systematic review: probiotics in the management of lower gastrointestinal symptoms in clinical practice — an evidence-based international guide. PUBMED https://www.ncbi.nlm.nih.gov/pubmed/23981066/
(13) Effect of probiotics on gastrointestinal symptoms and small intestinal permeability in children with atopic dermatitis. PUBMED https://www.ncbi.nlm.nih.gov/pubmed/15520759
(14) Probiotic supplementation affects markers of intestinal barrier, oxidation, and inflammation in trained men; a randomized, double-blinded, placebo-controlled trial. PUBMED https://www.ncbi.nlm.nih.gov/pubmed/22992437/
(15) Systematic review: probiotics in the management of lower gastrointestinal symptoms in clinical practice — an evidence-based international guide. PUBMED https://www.ncbi.nlm.nih.gov/pubmed/23981066/
(16) Composition and metabolism of the intestinal microbiota in consumers and non-consumers of yogurt. PUBMED https://www.ncbi.nlm.nih.gov/pubmed/17217568
(17) Contrasting effects of fresh and fermented kimchi consumption on gut microbiota composition and gene expression related to metabolic syndrome in obese Korean women. PUBMED https://www.ncbi.nlm.nih.gov/pubmed/25688926
(18) Intestinal permeability – a new target for disease prevention and therapy. PUBMED https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4253991/
(19) Impact of increasing fruit and vegetables and flavonoid intake on the human gut microbiota. PUBMED https://www.ncbi.nlm.nih.gov/pubmed/26757793
(20) Prebiotic fiber modulation of the gut microbiota improves risk factors for obesity and the metabolic syndrome. PUBMED https://www.ncbi.nlm.nih.gov/pubmed/22555633
(21) Intestinal Barrier Function: Molecular Regulation and Disease Pathogenesis. PUBMED https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4266989/
(22) Intestinal permeability, leaky gut, and intestinal disorders. PUBMED https://www.ncbi.nlm.nih.gov/pubmed/10980980
(23) Intestinal permeability in the pathogenesis of NSAID-induced enteropathy. PUBMED https://www.ncbi.nlm.nih.gov/pubmed/19148789
(24) The Leaky Gut and Altered Microbiome in Chronic Kidney Disease. PUBMED https://www.ncbi.nlm.nih.gov/pubmed/29056165
(25) Berberine improves intestinal epithelial tight junctions by upregulating A20 expression in IBS-D mice. PUBMED https://www.ncbi.nlm.nih.gov/pubmed/31306972
(26) Berberine alleviates dextran sodium sulfate-induced colitis by improving intestinal barrier function and reducing inflammation and oxidative stress. PUBMED https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5450762/
(27) Remodeling of Tight Junctions and Enhancement of Barrier Integrity of the CACO-2 Intestinal Epithelial Cell Layer by Micronutrients. PUBMED https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4520484/
(28) Aloe Vera for Tissue Engineering Applications. PUBMED https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5371879/
(29) Advances in treatment of ulcerative colitis with herbs: From bench to bedside. PUBMED https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4202341/
(30) Botanical Drugs as an Emerging Strategy in Inflammatory Bowel Disease: A Review. PUBMED https://www.ncbi.nlm.nih.gov/pmc/articles/PMC4630406/
(31) Prebiotic Potential of Herbal Medicines Used in Digestive Health and Disease. PUBMED https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6065514/
(32) Mucosal Barrier in Ulcerative Colitis and Crohn’s Disease. PUBMED https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3664489/
(33) Engineering the Mucus Barrier. PUBMED https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6463277/
(34) Intestinal epithelial cells in inflammatory bowel diseases. PUBMED https://www.ncbi.nlm.nih.gov/pmc/articles/PMC2937106/
(35) Gluten-Free Products for Celiac Susceptible People. PUBMED https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6304385/
(36) Dietary fiber and prebiotics and the gastrointestinal microbiota. PUBMED https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5390821/
(37) Long-Term Dietary Intake of Chia Seed Is Associated with Increased Bone Mineral Content and Improved Hepatic and Intestinal Morphology in Sprague-Dawley Rats. PUBMED https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6073254/
(38) High dose probiotic and prebiotic cotherapy for remission induction of active Crohn’s disease. PUBMED https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3725482/#B191-nutrients-05-01869
Leaky gut syndrome may in fact contribute to, and exacerbate multiple diseases, including autoimmune diseases such as inflammatory bowel disease, celiac disease, autoimmune hepatitis, type 1 diabetes, multiple sclerosis, and lupus
Leaky gut syndrome may in fact contribute to, and exacerbate multiple diseases, including autoimmune diseases such as inflammatory bowel disease, celiac disease, autoimmune hepatitis, type 1 diabetes, multiple sclerosis, and lupus.
Leaky Gut Syndrome Background
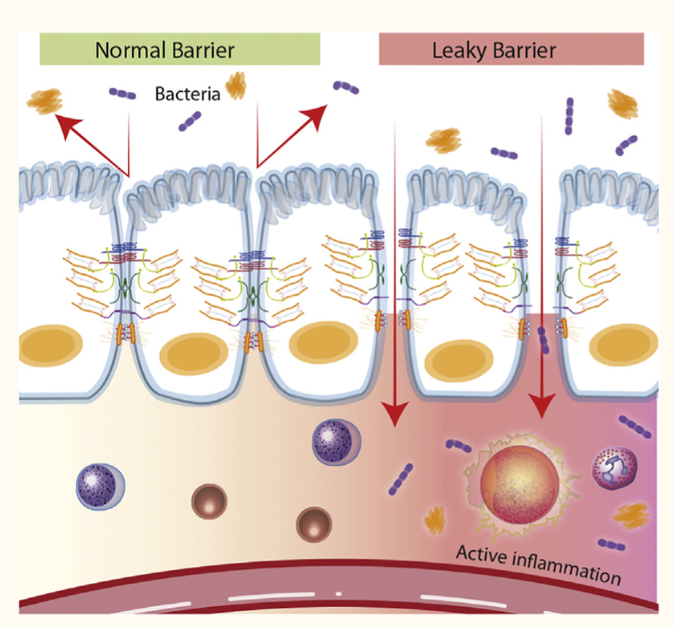
The human digestive tract is where food is broken down and nutrients are absorbed. The walls of the intestines act as barriers, controlling what enters the bloodstream to be transported to your organs. Small gaps in the intestinal wall called tight junctions allow water and nutrients to pass through, while blocking the passage of harmful substances. Intestinal permeability refers to how easily substances pass through the intestinal wall.
For digestion and absorption purposes, mammals have developed a very complicated and highly specialized gastrointestinal system maintained by what’s called the ‘mucosal barrier’. This gut membrane known as the intestinal epithelial lining, together with factors secreted from it, forms a barrier that separates our body from the environment. In disease conditions, the permeability of the epithelial lining may be compromised allowing the passage of toxins, antigens, and bacteria into the bloodstream creating a “leaky gut.” (1)
However, apart from absorbable nutrients, the intestinal mucosa also faces tremendous exterior antigens, including food antigens, commensal bacteria, pathogens, and toxins. Thus, a specialized barrier function is required to block the entry of diverse exterior antigens while absorbing nutrients. Impressively, in the intestine, the front line of this barrier is maintained by only a single layer of specialized epithelial cells that are linked together by tight junction proteins. Many other factors aid in support of this barrier including mucins, antimicrobial molecules, immunoglobulins, and cytokines. If any abnormalities occur among these factors, the intestinal permeability may increase, which is termed a “leaky gut.” (1)
Leaky Gut Syndrome Symptoms
When the tight junctions of intestinal walls become loose, the gut becomes more permeable, which may allow bacteria and toxins to pass from the gut into the bloodstream. This phenomenon is commonly referred to as “leaky gut.” When the gut is “leaky” and bacteria and toxins enter the bloodstream, it can cause widespread inflammation and possibly trigger a reaction from the immune system.
Supposed symptoms of leaky gut syndrome include bloating, food sensitivities, fatigue, digestive issues, and skin problems. However, leaky gut is not a recognized medical diagnosis. In fact, some medical professionals deny that it even exists. (6)
What Causes Leaky Gut Syndrome?
Numerous factors can affect gut permeability, such as various diet-derived compounds, alcohol consumption, and gut microbiota dysbiosis. Evidence suggests that gut microbiota is especially important in modulating gut permeability and intestinal barrier functions (1)
A large variety of external foreign substances colonize the gut, such as microorganisms, toxins, and antigens. Without an intact and properly functioning intestinal barrier, these substances can penetrate the tissues beneath the intestinal epithelial lining, diffuse into the blood and lymphatic circulations, and disrupt tissue balance. However, there is an efficient multifaceted intestinal barrier system with physical, biochemical, and immunological components that prevents the entry of most pathogens. These components coordinate with each other to prevent uncontrolled entry of gut contents to the internal body’s circulation. (1)
In order to understand the causes of leaky gut syndrome, let us first understand the main components comprising the intestinal barrier.
Understanding Leaky Gut Syndrome
There are several layers making up the intestinal musocal barrier, so let’s break these layers down a little to clearly understand how leaky gut comes about.
The Physical Gut Barrier
In humans, the intestinal epithelium covers a surface area as large as 400 m2. It can broken down as follows.
Intestinal cells
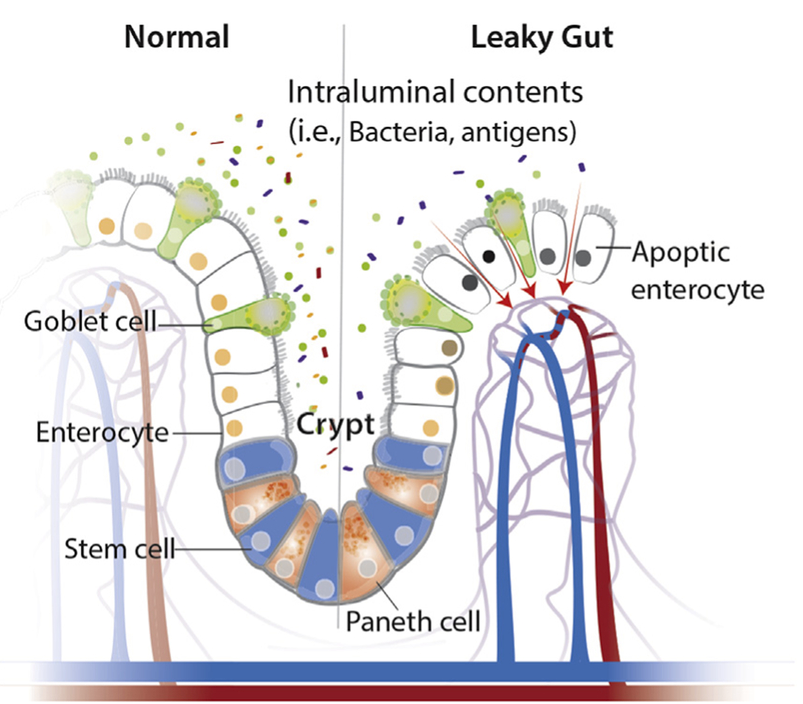
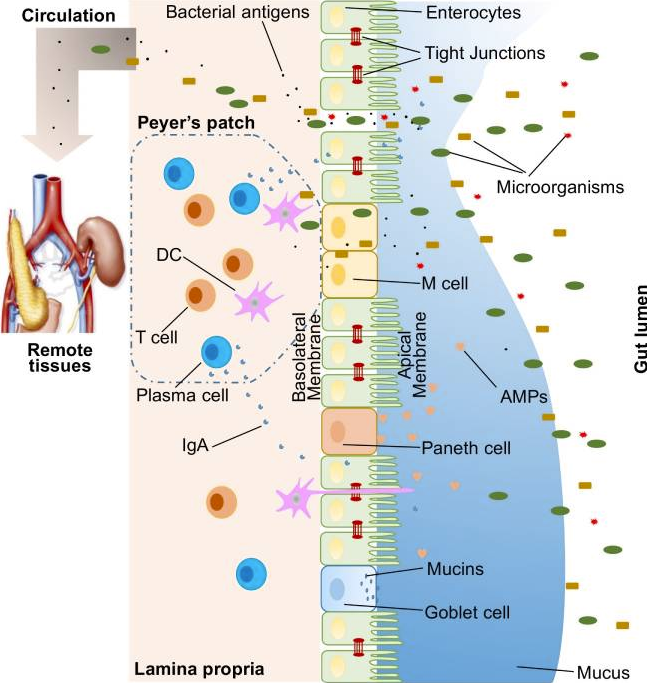
Though only a single layer of cells, the intestinal epithelial cells (IECs) are the mainstay of the intestinal barrier and serve as a physical barrier (see above). There are at least seven types of functional IECs—enterocytes, goblet cells, Paneth cells, microfold cells, enteroendocrine cells, cup cells, and tuft cells, although the functions of the last two cell populations are not well understood. Among all these cell types, enterocytes represent the absolute majority, accounting for at least 90%. All epithelial cell types originate from intestinal epithelial stem cells, and the turnover rate is high, as the cells are renewed every 3–5 days. (1)
Enteroendocrine, goblet and Paneth cells are the specialized, secretory epithelial cells that maintain the digestive or barrier function of the epithelium via hormone, mucin, and antimicrobial peptide secretion, respectively. (3)
Tight Junctions
This front line of barrier is only a single layer of specialized epithelial cells that are linked together by tight junction (TJ) proteins. A large variety of over 40 proteins, control the plasticity of these tight junctions. These transmembrane proteins interact with their partners on the opposing plasma membrane and provide a mechanical link between epithelial cells while establishing a diffusion barrier. If disrupted, permeation of potentially noxious molecules and organisms from the intestinal lumen can result in a cascade of events including immune activation and inflammation, eventually triggering the development of intestinal and systemic diseases. (3)
Mucous Lining
On top of the gut epithelium, there are two layers of mucus, the inner and outer layers, that cover the whole intestinal epithelial lining and provide physical protection. Goblet cells are the central cell type for the formation of mucus. The mucus lining contains diverse molecules including Immunoglobulin A (IgA) as well as enzymes and proteins, such as lactoferrin. (1) IgA is the first line of defencein the resistance against infection, via inhibiting bacterial and viral adhesion to epithelial cells and by neutralisation of bacterial toxins and virus, both extra- and intracellularly. (2) Colitis would spontaneously develop in Mucus deficient mice, indicating a critical role for mucosal protection. (1)
Gut Microbiome
The healthy gut bacteria have been described as one component of the intestinal physical barrier primarily due to its two major functions. The first is to promote resistance to the colonization of harmful or pathogenic bacteria species by competing for nutrients, occupying attachment sites, and releasing antimicrobial substances. Additionally, the gut microbiota regulates the digestion and absorption of nutrients to supply energy to epithelial cells, which are a major component of the physical barrier. Certain microbiota, bacterial products, and metabolites affect the intestinal barrier function and are responsible for the subsequent breakdown of tissue homeostasis.
When there is a leaky gut, commensal bacteria in gut lumen, together with their products, are able to escape the lumen of the gut, which may induce inflammation and cause systemic tissue damages if translocated into peripheral circulation. This process of translocation is called microbial translocation. The important role of gut microbiota in modulating mucin production from goblet cells is further evidenced in animals with lower loads of bacteria. The thinner mucus layers would allow for bacteria penetration, which may initiate inflammation and inflammatory diseases such as colitis. A balance exists between commensal bacteria and the mucus layers, and together they contribute to the maintenance of gut homeostasis. (1)
The Biochemical Gut Barrier
Biochemical molecules with antimicrobial properties exist in the mucus as well as far into the lumen and include bile acids and antimicrobial proteins. These diverse molecules form a complicated network to reduce the load of colonized bacteria and decrease the chance of contact between luminal antigens and host cells.
The Immunological Gut Barrier
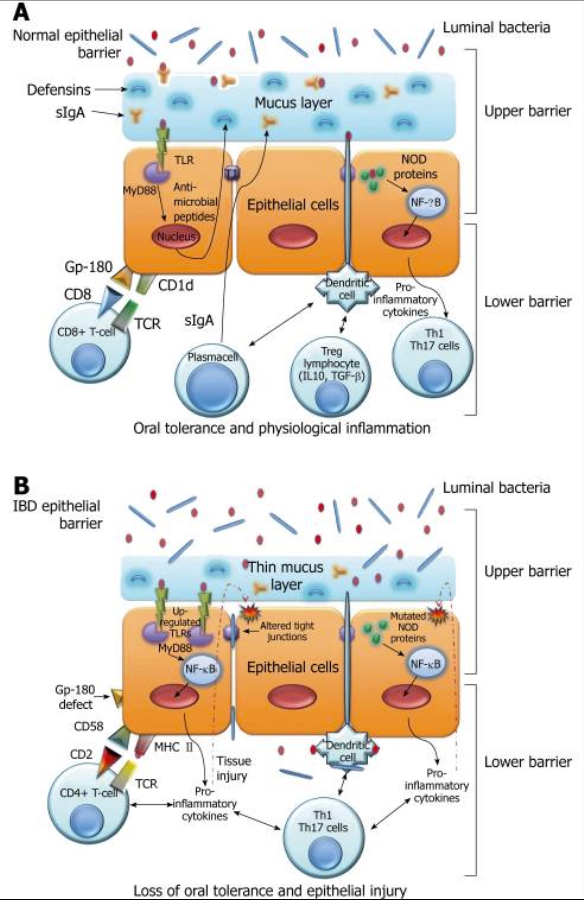
Below the intestinal epithelium layer, there are organized lymphatic tissue containing a variety of immune cells, including B cells, T cells, dendritic cells, and neutrophils, orchestrate the immune response. In addition, goblet cells are capable of sensing invasive pathogens and inhibiting the entry of pathogenic bacteria into the host immune system. Another component of the immunological barrier is secretory IgA. As the most abundant immunoglobulin in the body, IgA resides primarily on intestinal mucosal surfaces.
Autoimmune disorders are characterized by the generation of autoantibodies against self-antigens that attack the body’s own tissues, resulting in damage. Genetic and environmental triggers have been long known as the major contributors to the development of autoimmunity. Increasing evidence in recent years suggests that microbial translocation and intestinal barrier dysfunction, which may be affected by gut microbiota, are another important causative element for autoimmune disorders. (1)
Causes of Leaky Gut Syndrome
A large variety of gut barrier disruptors and/or gut microbiota disturbers may potentially result in microbial translocation and subsequent inflammation locally and systemically. These include diet, infections, alcohol consumption, and burn injury.
Diet and Leaky Gut Syndrome
Nutrients and food ingredients have been reported to contribute to the maintenance or alterations of gut microbiota and the intestinal barrier function. In one recent study a low-fiber diet consumption was found to trigger the expansion of mucus-degrading bacteria. As a result, the thickness of mucus is significantly decreased in mice fed with fiber-deficient diets. The thinner mucus and compromised intestinal barrier function lead to a higher susceptibility to certain colitis-causing pathogens.
Moreover, a diet high in saturated fat has been shown to greatly decrease Lactobacillus and increase Oscillibacter, and these changes were correlated with significantly increased permeability in the proximal colon. (1)
Chronic alcohol consumption is responsible for intestinal barrier dysfunction, alterations on both the quality and quantity of gut microbiota. Interestingly, probiotic Lactobacillus is significantly suppressed during alcohol consumption. (1) It is postulated that alcohol-induced intestinal permeability facilitates enhanced translocation of endotoxin to distant organs leading to inflammation and tissue damage. Similar lesions have been found in healthy volunteers and active alcoholics following acute alcohol consumption and plasma endotoxin levels in alcoholics were found to be 5-fold greater than healthy controls. (4)
Acid Load and Leaky Gut
Chronic kidney disease results in disruption of the intestinal epithelial barrier as well as profound changes in the gut microbial flora. These events are largely mediated by (1) heavy influx of circulating urea into the gut (2) a diet low in fruits and vegetable (alkaline foods). (3) and a diet low in symbiotic or probiotic foods, like yoghurt. Collectively, these factors promote systemic inflammation and cardiovascular morbidity by mediating microbial dysbiosis, disruption of the intestinal epithelial barrier, and translocation of endotoxin, bacterial fragments, and uremic toxins across the “leaky gut” into the bloodstream. (24)
Bacterial Infections and Leaky Gut Syndrome
Infections can play a role in regulating the mucosal barrier.
- A good example is Helicobacter pylori, a Gram-negative bacterium infecting the human stomach. H. pylori is known to directly increase epithelial permeability.
- When rats were given a bacterial cocktail containing Salmonella, disruption of the intestinal barrier integrity was observed. (1)
- Cholera is another major enteric pathogen that alters intestinal barrier function through the disruption of TJs, dysregulation of intestinal ion and fluid transport and the initiation of inflammatory cascades. (4)
- E-Coli is a diarrhea-causing bacteria that disrupts Tight Junction proteins (TJs) by adhering directly to the surface of epithelial cells. (4)
- While many bacterial products have been demonstrated to alter TJs, the enterotoxin of Clostridium perfringens, which is a common cause of food poisoning, directly interacts with and utilizes TJs as receptors. (4)
- The results of one study with mice suggested that gastrointestinal Candida colonisation promotes sensitisation against food antigens, at least partly due to mast cell mediated hyperpermeability in the gastrointestinal mucosa. (5)
Stress and Leaky Gut Syndrome
A variety of stressors (physiologic, pharmacological, psychologic, and others) affect the intestinal barrier and have been studied using human and animal models. Moderate exercise has been shown to increase gut permeability. Shunting of the blood from visceral tissues to more active tissues during exercise can therefore result in varying degrees of intestinal damage including alterations in permeability. This is similar to effect of the adrenal cortisone ‘fight or flight’ stress response and appears to be intensity dependant. (3)
Pharmaceuticals and Leaky Gut Syndrome
Non-steroidal anti-inflammatory drug use is associated with a high incidence of GI side effects, and there is substantial evidence indicating that chronic use can alter intestinal barrier function and cause significant GI damage, including ulcers, perforation, hemorrhage and an exacerbation of IBD. Non-steroidal anti-inflammatory drugs can also uncouple mitochondrial oxidative phosphorylation, which impairs the mitochondrial energy production necessary for tight junction complex integrity leading to increased intestinal inflammation and permeability. (4)
Food Allergies and Leaky Gut Syndrome
Food allergies are adverse, immune-mediated reactions to ingested food proteins/antigens. It is hypothesized that intestinal barrier dysfunction may contribute to both antigen sensitization and also the anaphylactic immune phase of disease. The development of food allergies is dependent on the exposure of the food antigen to the mucosal immune system, which leads to antigen sensitization and the production of dietary antigen-specific cells. It is hypothesized that altered intestinal barrier function permits increased dietary antigen transport across the intestinal barrier and exposure of dietary antigens to the mucosal immune system leading to the development of the dietary antigen-specific response. (4)
Irritable Bowel Syndrome and Leaky Gut
The IBDs, Crohn’s disease and ulcerative colitis, are chronic, relapsing-remitting inflammatory diseases. An emerging model of the pathogenesis of IBD suggests there are three essential factors: 1) a breakdown in intestinal barrier function; 2) exposure of luminal contents to immune cells in the lamina propria; and 3) an exaggerated immune response. There is a growing body of data to suggest that increased intestinal permeability is a primary etiologic factor contributing to IBD pathogenesis. (4)
Leaky Gut Syndrome Natural Treatment
It would appear from the research above that leaky gut syndrome is caused by a combination of events. Poor diet, stress along with long-term ingestion and exposure to toxic irritants, pathogens and detrimental microbes all contribute to the onset of a leaky gut.
The restoration of a healthy gut environment will be the most relaible way to encourage the body to heal the damage cuased by the above.
Here are a few strategies to support a healthy gut:
Limit your refined carb intake
Harmful bacteria thrive on sugar, and excessive sugar intake can harm gut barrier function (7, 8, 9). The simplest way to achieve this is to embark on a wholefood diet, completely avoiding processed foods. This includes bread, pasta, cerelas, snack bars, sugary drinks and all manner of sweets, pastries, cakes, muffins or other types of baking.
Take a probiotic supplement: Probiotics are beneficial bacteria that can improve your gut health. Probiotic supplements have been shown to be beneficial for gastrointestinal diseases (10, 11, 12, 13, 14). Plain unsweetened yoghurt as part of your regular diet is suggested, along with a regular dose o a good probiotic strain such as L-Reuteri.
Eat fermented foods: Fermented foods, such as plain yogurt, kimchi, sauerkraut, kefir and kombucha, contain probiotics that can improve gut health (15, 16, 17) See a couple of recipes here including Fermented Vegetables – Sauerkraut and Yoghurt Dessert
Eat plenty of high-fiber foods: Soluble fiber, which is found in fruits, vegetables and legumes, feed the beneficial bacteria in your gut (18, 19, 20). The simplest way to achieve this is to look at a raw food diet. It is hard to eat losts of raw without consuming large quantities of fibre in the process. Read more about raw food
Limit the use of NSAIDs: The long-term use of NSAIDs like ibuprofen contributes to leaky gut syndrome (21, 22, 23)
Introduce a more alkaline diet, reducing the acid load on kidney function. (24) Acid forming foods include meats, hard cheeses, sugar and nuts. Alkaline foods include virtually all fresh fruits and vegetables. Read more about alkaline diet here
Herbs for Leaky Gut Syndrome
Golden Seal
Berberine, the main active contistuent in Golden seal downregulates abnormal activation of the TNF-α-NF-κB-MLCK pathway by upregulating expression of A20 in a mouse model of IBS-D, thereby protecting intestinal epithelial tight junctions and repairing the damage IBS-D causes to the intestinal epithelial barrier. (25)
Data from another study suggests that berberine alleviates colitis principally by improving intestinal barrier function and promoting anti-inflammatory and antioxidative stress responses. In turn these effects inhibit macrophage infiltration into the colon and thus may be central to the anti-colitis activity of berberine. (26)
The plant alkaloid, berberine, was also earlier reported to improve barrier properties of cell layers. Berberine also protects against TNF-alpha-induced barrier compromise of epithelial layers as well as rat colon mucosa. (27)
Aloe Vera
Aloe vera is one of the oldest medicinal plants on record due to its biological properties and health benefits. Aloe has been described as having anti-inflammatory effects, wound healing properties, radiation damage repair benefits, antibacterial, antiviral, antifungal, antidiabetic, and antineoplastic activities, hematopoietic stimulation, and antioxidant effects. (28)
The clinical value of aloe vera gel has been assessed. In a randomized, double-blind, placebo-controlled trial, 44 hospitalized patients with mild or moderate Ulerative Colitis (UC) received oral aloe vera gel treatment or placebo, 200 mL daily for 4 wk. Clinical remission, improvement and response of the disease had been observed in 9 (30%), 11 (37%) and 14 (47%), respectively, of 30 UC patients taking aloe vera, compared to one (7%), one (7%), and two (14%), respectively, of 14 UC patients receiving placebo. The clinical colitis activity index and histological scores of the patients decreased significantly during treatment with aloe vera (P = 0.01 and P = 0.03, respectively), but not with placebo. Endoscopic score and laboratory variables displayed no significant differences in both groups of patients with aloe vera or placebo treatment. Side events were minimal and similar between aloe vera and placebo. (29)
Among the different components of the gel, which comprise acetylated mannans, polymannans, anthraquinone C-glycosides, anthrones, anthraquinones (emodin), and lectins, with reputed biological activities, in the same study it was proposed that the chromone aloesin seemed to be essential for the control of the intestinal inflammatory process. (30)
Licorice and Slippery Elm
Studies highlighting the significant prebiotic potential of Licorice and Slippery Elm and suggest that the health benefits of these herbs are due, at least in part, to their ability to modulate the gut microbiota in a manner predicted to improve colonic epithelium function, reduce inflammation, and promote protection from bacterial opportunistic pathogenic infection. (31)
Plant Enzymes and Leaky Gut Syndrome
The intestinal mucosa produces antimicrobial peptides, called defensins, which contribute to maintaining host immunity and protect from pathological flora. These defensin peptides are produced by enzymatic digestion to turn them into their active form. This process is necessary to allow a conformational folding of these proteins, helpful to accomplish their function. (33)
Evidence demonstrates that in CD there is a defective expression and release of non-functional peptide that forms an unfolded structure, probably due to a defective enzymatic digestion. A decreased concentration of defensins is responsible for the presence of a less efficient mucus membrane as a biochemical barrier for pathogenic bacteria. (33)
The mucus layer in the gastrointestinal tract plays important role in host innate defense, regulation of secretion, and absorption processes, maintaining colonization resistance, which composes the integrity of protective mucus barrier in the large intestine. Different types of mucins synthesis, secretion, and expression were found in patients with Ulceratice Colitis (UC) and Crohn’s Disease (CD). The expression of mucins correlated with the activity of disease and the extent of the inflammatory process in the large intestine. The most pronounced alteration of mucins expression was observed in patients with severe UC and CD. (32)
As mucus is a complex network of crosslinked proteins. Proteolytic enzymes play a major role in the natural turnover of the mucus layer. For example, proteolytic enzymes (papain, and bromelain are naturally found in the digestive tract, papaya, and pineapple, respectively added to native pig intestinal mucus for 12 hours at 37 °C had variable efficiency in reducing viscosity of the mucus network. (32)
Enzymes and Gluten Intolerance
The enzymes obtained from various sources have been used for modifying the immunogenic fraction of gluten protein. The modified gluten network after endopeptidase treatment reduces the technological properties (viscoelasticity) of dough and baked products, which are supplemented by structuring agents such as pre-gelatinized starch, emulsifiers and hydrocolloids. The peptidases other than digestive enzymes exhibited predominantly post-proline and/or post-glutamine cleavage activities, effectively degrading the 33-mer peptide into fragments of <9 amino acids in length. The endopeptidase originating from bacterial origin completely degrades the gluten-immune toxic peptides during the preparation of wheat flour dough. (35)
Prebiotic Fibre and Leaky Gut Syndrome
Increasingly, diet is recognized as a key environmental factor that mediates the composition and metabolic function of the gastrointestinal microbiota. Indeed, consumption of specific dietary ingredients, such as fiber and prebiotics, is an avenue by which the microbiota can be modulated. Consumption of dietary fiber promotes extensive metabolic interactions among bacterial species present in the gastrointestinal microbial community. Bacterial fermentation of fibre results in the production of acidic fermentation end products, primarily lactic acid and SCFAs, that reduce the colonic pH, which in turn improves the composition of the microbial communities present in the gastrointestinal tract. Responses are also dependent on fiber intake in the context of the entire diet; for example, dietary fiber per kilocalorie has been shown to be positively related to both Bifidobacterium spp. abundances and fecal butyrate concentrations. (36)
Chia seed contains soluble fiber, which may have improved the time of food digestion and the absorption of nutrients by intestinal cells. The physical and chemical characteristics of soluble fiber may alter the absorption of food nutrients, such as fermentation, bulking and binding ability, viscosity and gel formation, water-holding capacity, and solubility. (37)
Pslyllium Husk contains soluble fibre and has been shown to reduce inflammatory bowel disease when combined with other probiotics. (38)
A Herbal Program for Leaky Guy Syndrome
By combining all of these recomendations into one simple program the Ultimate Herbal Detox program is deisgned to resolve the imbalance of gut microbiome, feed prebiotic fibre, alkalise the diet while simultaneoulsy provding all of the herbs mentioned above.